Introduction
Enzymes are molecules that regulate the chemical reactions that occur in all living organisms. Almost all enzymes are globular proteins that act ascatalysts,substances that speed up chemical reactions. Enzymes catalyze reactions by reducing the activation energy for a specific reaction to occur and yet are neither destroyed nor altered during this process. At the molecular level, enzymes catalyze these reactions by briefly binding to the substrate or reactants to form an enzyme-substrate complex. The reaction takes place while the substrate is bound to the enzyme, converting the substrate to the new product. The new product is then released from the enzyme substrate complex and the enzyme is then free to bind with more substrate.
基于这个模型,产品的速度can be produced depends on the amount of enzyme and substrate that are present during the reaction. If there is excess substrate and a small amount of enzyme in solution, the reaction rate, or velocity of the reaction, will increase with the amount of enzyme in the solution. In this case, all of the enzyme molecules are busy catalyzing reactions even though there is still plenty of substrate that can be turned into product. The velocity of the reaction can only increase if the concentration of enzyme is increased. Put another way, the velocity of the reaction should increase in direct proportion to the concentration of enzyme in solution.
Objectives
In this experiment, you will
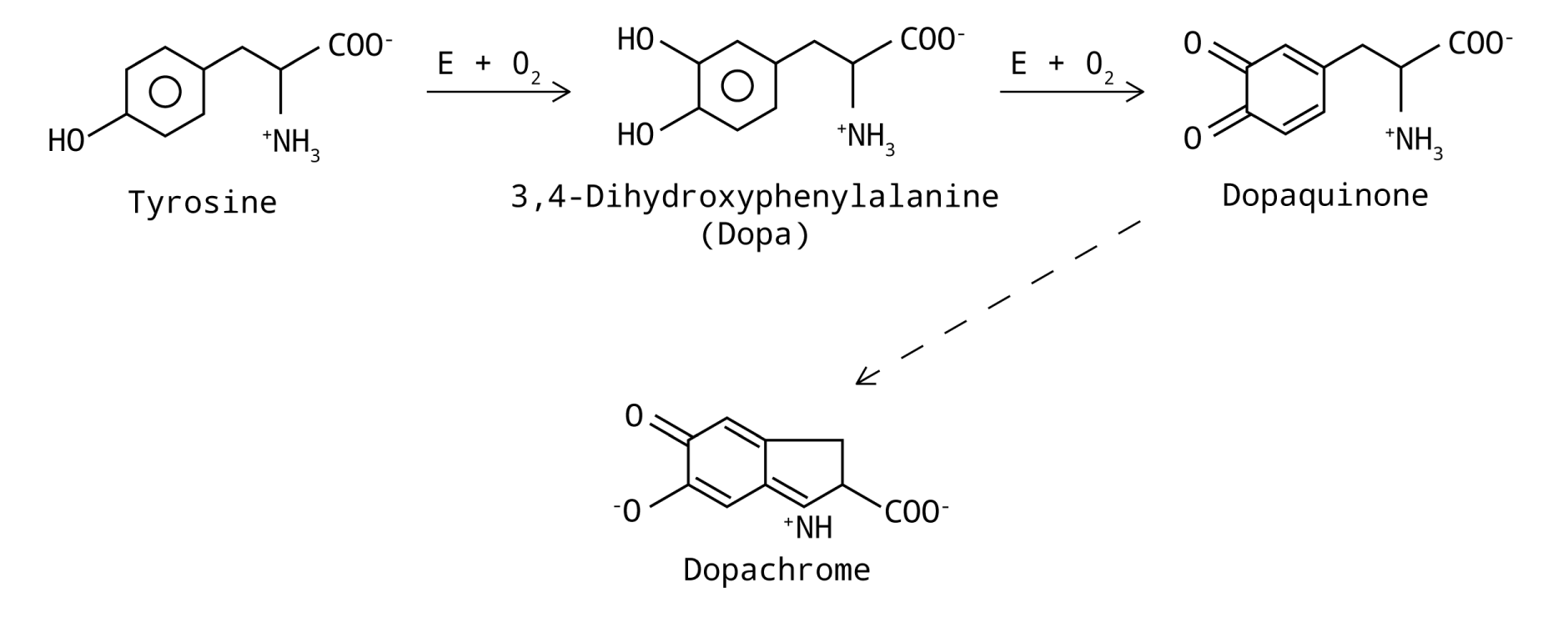
- Observe and compare the reaction rate of two substrates, tyrosine and DOPA.
- Determine the effect of increasing enzyme concentration on the reaction rate of an enzyme at a given substrate concentration.
- Determine the effect of increasing substrate concentration on the reaction rate of an enzyme at a given enzyme concentration.
- Estimate the parameters,Vmax, ½Vmax, andKmfor your enzyme extract.